Polycarbonates
Properties
Polycarbonates are formed from the reaction of an (aromatic) dihydroxy compound and phosgene with the elimination of hydrogen chloride. The most important diol is bisphenol A (BPA). Many other diols have been tested in place of bisphenol A, but only a few have found commercial use. For example 1,1-bis(4-hydroxyphenyl)cyclohexane is used as a comonomer to suppress the crystallisation of bisphenol A carbonate products. Another comonomer is tetrabromobisphenol A, which is randomly or block copolymerized with BPA to improve the fire resistance of Bis A polycarbonates. The monomer tetramethylcyclobutanediol has been investigated as a potential replacement for BPA.
Polycarbonates (PC) are one of the largest sales volume engineering thermoplastics. The anual production exceeded 3.75 million tons in 2012. They are amorphous polymers that can be easily molded and thermoformed, and have many attractive properties. For example, PCs have an exceptional high light transmittance and impact toughness as well as good dimensional stability and creep resistance. But they also have some disatvantages. Among these are poor scratch and solvent resistance and a tendency to yellow when exposed to UV light for a prolonged time. To improve the scratch and solvent resistance, polycarbonates are frequently coated with silicones. The tendency to yellow has been greatly reduced with UV absorbers such as benzotriazoles and hydroxyphenyltriazines.
To lower the costs and to improve certain properties, PCs are sometimes blended with a number of other polymers including ABS rubber, PET, PBT, and styrene-maleic anhydride copolymer (SMA). PET and PBT are added to improve the chemical resistance and processability, ABS imparts improved processability and low-temperature properties, and SMA improves the temperature stability.
Recently, aliphatic polycarbonates have become available.
These polymers are produced by ring opening polymerization of cyclic
carbonates or through the copolymerization of CO2 with one or more epoxides.
Of commercial importance are polypropylene carbonate (PPC), polyethylene carbonate
(PEC), and poly(cyclohexene carbonate). Among these, poly(propylene
carbonate) has the largest sales volume. It is an amorphous
polymer with a Tg between 25 and 45°C. it has a high gas
permeability, excellent adhesion properties and good lubricity.
COMMERCIAL Polycarbonates
Commercial grades of unfilled and glass-reinforced polycarbonates are available from many companies. Three major manufacturers are Bayer, Styron and SABIC. The non-reinforced, general purpose and food contact grades are available in several different viscosity classes. Aliphatic polycarbonates are produced on a much smaller scale. Important manufacturers include Perstop, and Empower Materials. Some of the most common aromatic and aliphatic polycarbonates are shown below.
| Polycarbonate | Structure of Repeat Unit | Trade Name |
| Poly(bisphenol-A-carbonate) |
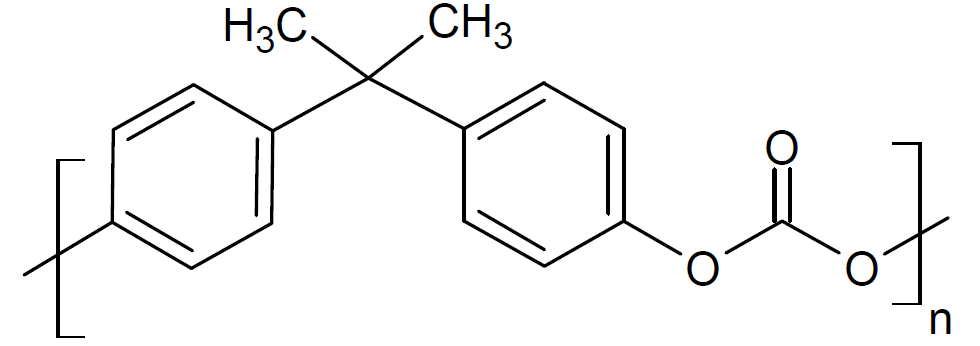
| Makrolon®, Lexan™ |
| Poly(propylene carbonate) |
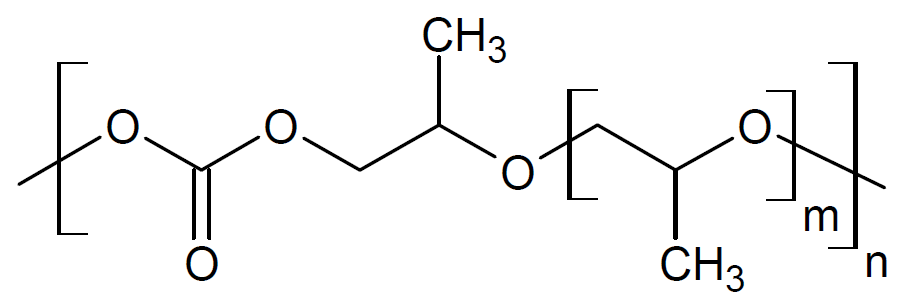
| QPAC® 40 |
| Poly(cyclohexene propylene carbonate) |
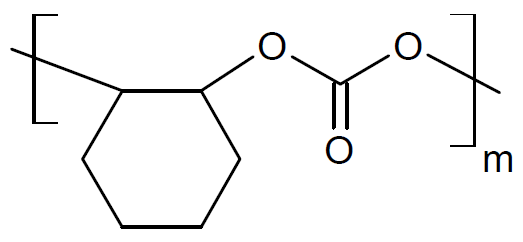
| QPAC® 130 |
Applications
Because of its exceptional transparency, impact resistance and dimensional stability, polycarbonate finds uses in many industries. Important applications include safety helmets, compact discs, power tools, appliance housings, glazing for windows, doors, face shields, sun glasses, and automotive parts such as instrument and exterior panels, and wheel covers.
Aliphatic polycarbonates are mainly used as toughening agents in adhesives, as chain extenders in urethanes, and as sacrificial binders in ceramic compositions which decompose and evaporate prior sintering. Other noteworthy applications include 3D printing of ceramics, thick film pastes, and biodegradable plastics (PPC/starch blends).